Q1. A radioactive uncleus undergoes a series of decay according to the scheme
 If the mass number and atoms number of A are 180 and 72 respectively, what are there number for A4?
If the mass number and atoms number of A are 180 and 72 respectively, what are there number for A4?
 If the mass number and atoms number of A are 180 and 72 respectively, what are there number for A4?
If the mass number and atoms number of A are 180 and 72 respectively, what are there number for A4?Solution

Q2. The ground state energy of hydrogen atom is -13.6eV
(i) What is the kinetic energy of an electron in the second excited state ?
(ii) If the electron jumps to the ground state from the second excited state, calculate the wavelength of the spectral line emitted.
OR


Solution
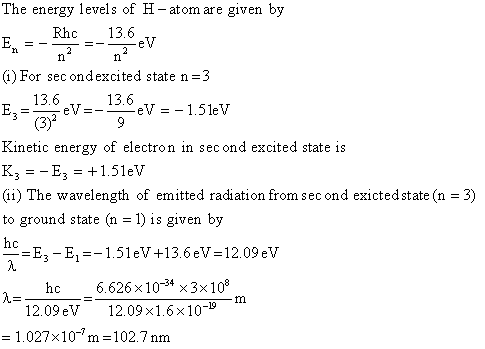 OR
OR
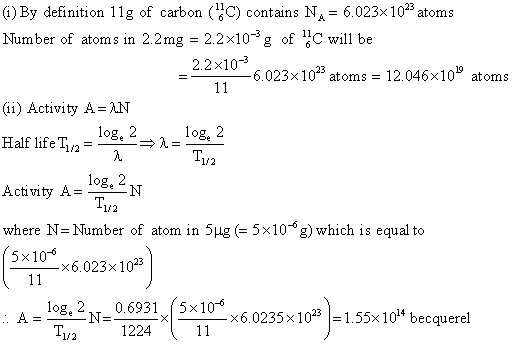
Q3. Give the mass number and atomic number of elements on the right hand side of the decay process.

Solution
The complete equation representing mass number and atomic number is given below
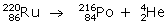 For Polonium Z=84, A=216
For Helium Z=2, A=4
For Polonium Z=84, A=216
For Helium Z=2, A=4
Q4.
Complete the following nuclear reactions
(a) 4Be9 + 1H1 →3Li6 + ................
(b) 5B10 + 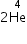 →
→  + ..............
+ ..............
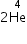 →
→  + ..............
+ ..............
Solution
(a) 4Be9 + 1H1 →3Li6 + 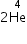 (b) 5B10 +
(b) 5B10 + 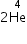 →
→ + on1
+ on1
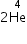 (b) 5B10 +
(b) 5B10 + 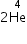 →
→ + on1
+ on1
Q5. The isotopes 8O16 has 8 protons, 8 neutrons and 8 electrons, while 4Be8 has 4 protons, 4 neutrons and 4 electrons. Yet the ratio of their atomic masses is not exactly 2. Why?
Solution
The ratio of mass of 8O16 and mass of 4Be8 is not exactly two because of difference in the mass defect/binding energy of the atomic nuclei of the two elements.
Q6. A radioactive sample contains 2.2 mg of pure  which has half life period of 1224 seconds. Calculate
(i) the number of atoms present initially.
(ii) the activity when 5μg of the sample will be left.
which has half life period of 1224 seconds. Calculate
(i) the number of atoms present initially.
(ii) the activity when 5μg of the sample will be left.
 which has half life period of 1224 seconds. Calculate
(i) the number of atoms present initially.
(ii) the activity when 5μg of the sample will be left.
which has half life period of 1224 seconds. Calculate
(i) the number of atoms present initially.
(ii) the activity when 5μg of the sample will be left.Solution
(i) By definition 11g of carbon  contains NA = 6.023 x 1023 atoms
number of atoms in 2.2 mg = 2.2 x 10-3g of
contains NA = 6.023 x 1023 atoms
number of atoms in 2.2 mg = 2.2 x 10-3g of  will be
=
will be
=  (ii) Activity A = λN
Half life T1/2 =
(ii) Activity A = λN
Half life T1/2 = Activity A =
Activity A =  Where N = Number of atoms in 5μg (= 5 x 10-6g) which is equal to
Where N = Number of atoms in 5μg (= 5 x 10-6g) which is equal to


 contains NA = 6.023 x 1023 atoms
number of atoms in 2.2 mg = 2.2 x 10-3g of
contains NA = 6.023 x 1023 atoms
number of atoms in 2.2 mg = 2.2 x 10-3g of  will be
=
will be
=  (ii) Activity A = λN
Half life T1/2 =
(ii) Activity A = λN
Half life T1/2 = Activity A =
Activity A =  Where N = Number of atoms in 5μg (= 5 x 10-6g) which is equal to
Where N = Number of atoms in 5μg (= 5 x 10-6g) which is equal to


Q7. What will be the ratio of radii of two nuclei of mass number A1 and A2?
Solution
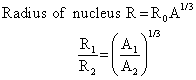
Q8. The half life period of a radioactive substance is 30 days. What is the time for ¾ th of its original mass to disintegrate?
Solution
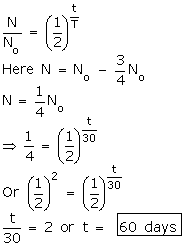
Comments
Post a Comment